R&D Lab & Manufacturing Plant
- System Scope: Validation includes ERP Systems, DMS, Network & PC-based Systems, Facility/Utility/Barcode/Warehouse Systems, Building Automation, Maintenance Management Systems, SCADA/PLC/DCS/MES/EBRS systems, and process equipment.
- Clinical Systems: Validation of eCRF, eSD, eTMF, Patient Databases, IV/WRS systems, data migration plans, and data quality control.
Deliverables:
- Validation Master Plan (VMP)
- SOPs for CSV
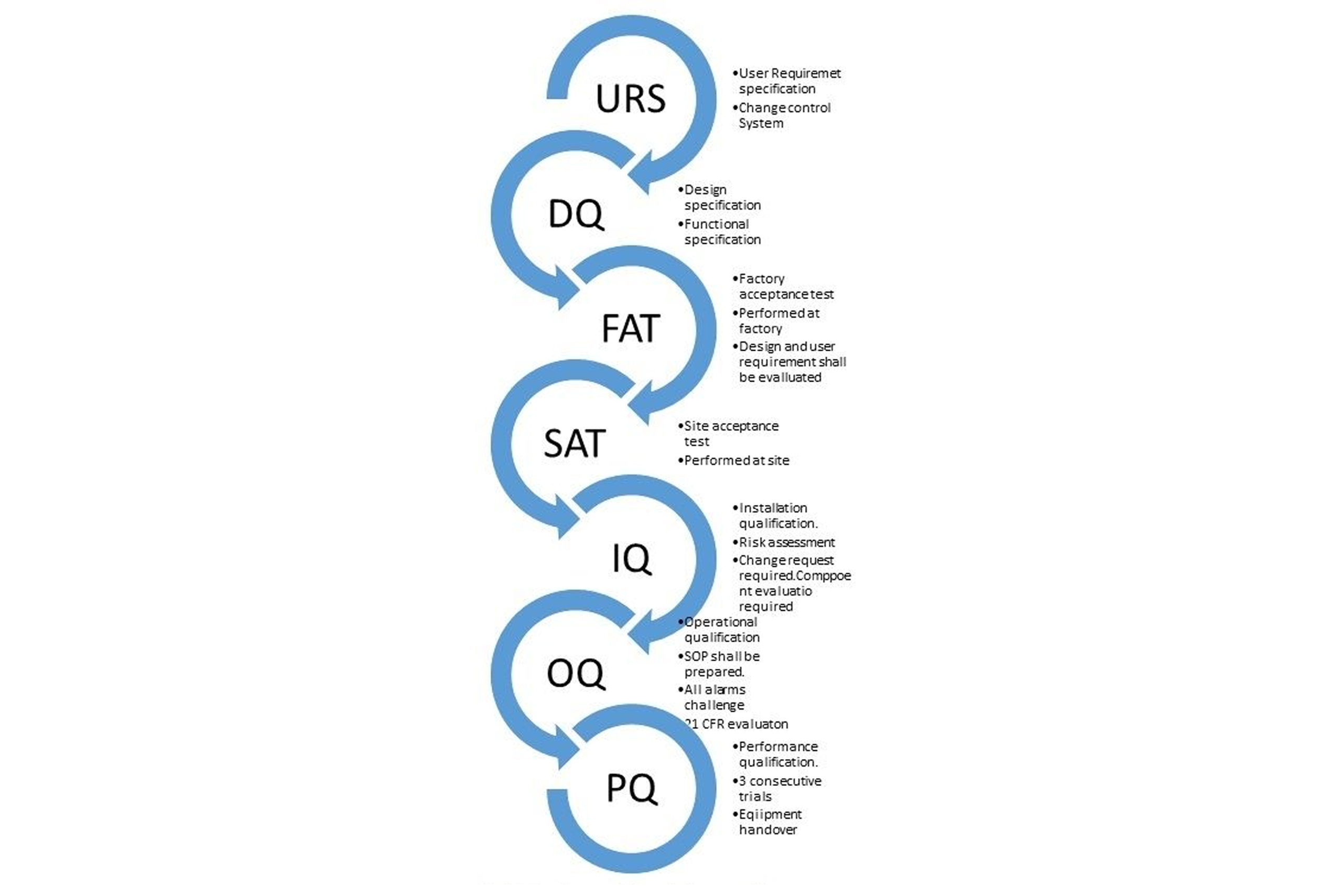
- URS/SRS – Requirement Specifications
- Design & Configuration Specs (DCS)
- Risk Assessments (QRM/RA, FRA)
- IQ/OQ/IOQ (Installation & Operational Qualifications)
- RTM (Requirement Traceability Matrix)
- Validation Summary Report (VSR)
- Audit trails, logical security, access control, data integrity assessments (DIRA Audit)
- Spreadsheet (Excel) validation
Infrastructure Support:
- Workforce augmentation
- IT Infrastructure validation
- Execution & Approval support
- Data Management Systems
- Compliant with: 21 CFR Part 11, EU Annex 11, PIC/S, ANVISA, GAMP 5, ISO 14971, ISO 27001, ISO12207, ASTM E2500
Validated Systems Include:
Lab Equipment
- HPLC, GC, FTIR, TOC, Stability Chambers, Muffle Furnace, Humidity Chambers
- Polarimeter, Dissolution Tester, Sonicator, Refrigerator
Manufacturing Equipment
- BMS/EMS
- Water Systems (WFI/PW/EDI)
- Blenders, FBP/FBD, Compression Machines
- Rapid Mixer Granulators, Compactors
- Strip/Blister Packing Machines
- Vial Washing, Filling, Sealing Machines
- De pyrogenation Tunnel, Isolators, Lyophilizers
- ORABS, Manufacturing Vessels, Integrity Apparatus
Application Categories (GAMP 5)
- Category 1: Infrastructure Software
- Category 3: Non-configured (firmware, instruments)
- Category 4: Configured Software (SCADA, ERP, BMS, LIMS)
- Category 5: Custom Software (internal/external IT apps, macros, PLC logic)
Approaches:
- Initial Qualification: Risk Assessment (QRM/RA), IQ, OQ, Validation Summary Report
- Periodic/Re-Qualification: Re-IQ & OQ based on client policy, with validation summary
- Addendum-Based Approach: For updates/modifications in hardware/software. Includes documentation and re-validation.
Validation Deliverables (as applicable to PLC, SCADA, Firmware):
- Design & Functional Specifications (DQ/SDS/FDS)
- Quality Risk Management (RA/QRM)
- IQ/OQ/IOQ with detailed test scripts
- RTM for mapping requirements to test results
- VSR covering lifecycle, deviations, incidents
- Gap Assessment, GxP Compliance Check, and Data Integrity Risk Assessment.