Regulatory Strategy & Global Market Approvals
- Pre-market Notification (510k) Consulting
- India CDSCO Regulatory Approval Services
- CDSCO Import Authorization Services
- CDSCO Factory License Assistance
- CE Certification under EU MDR
- UK Market Access via UKCA Certification
- Russian Market Approval for Devices
- SFDA Certification for Medical Devices (KSA)
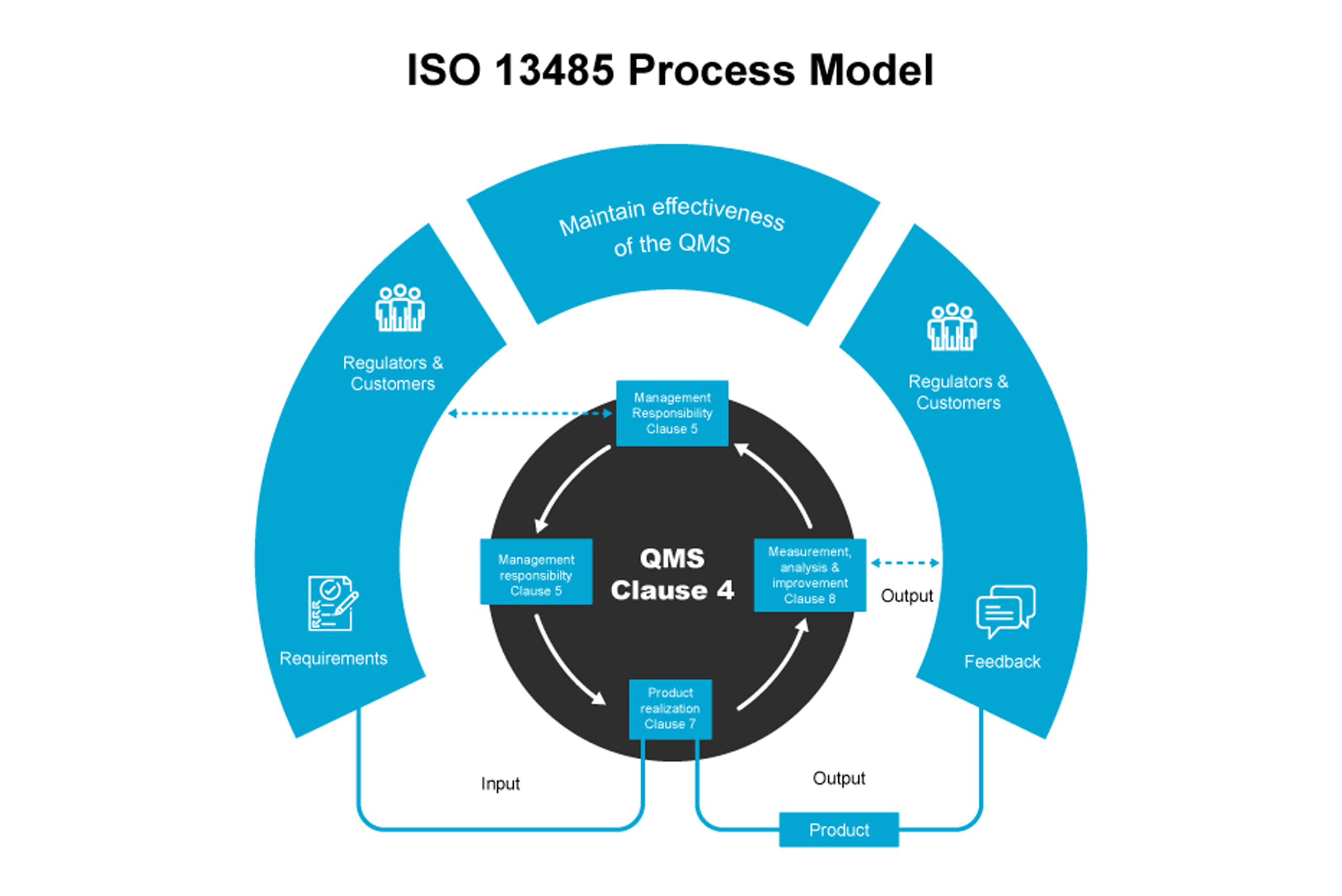
ISO 13485-Based Quality Management Solutions
- FDA 21 CFR PART 820 QUALITY SYSTEM REGULATION
- MEDICAL DEVICE
- QMS SOFTWARE (EQMS)
- ISO 15378:2017
- CERTIFICATION
- PRIMARY PACKAGING
- REGULATORY
- CONSULTING
- MDSAP
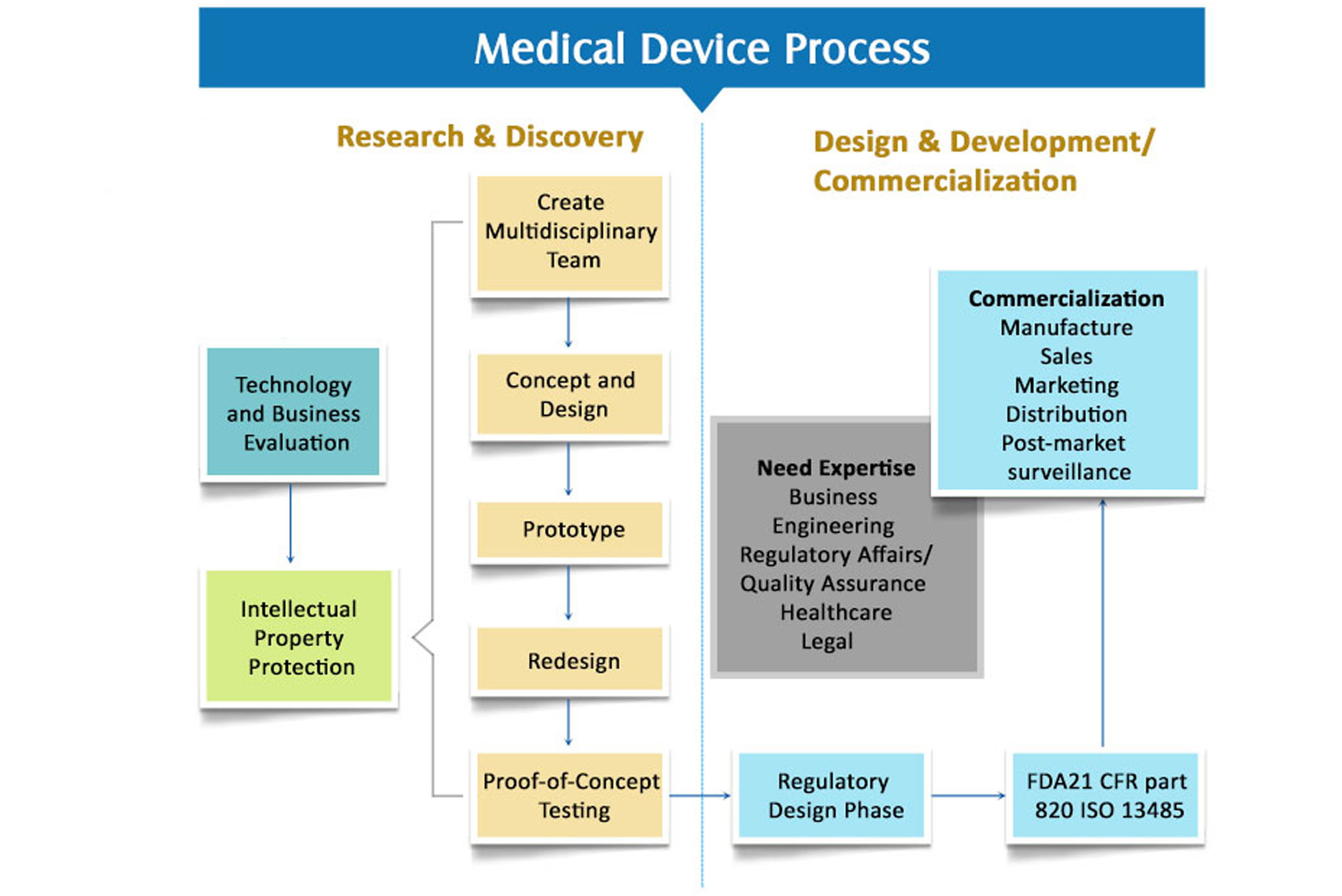
- Medical Device QMS Setup & Compliance
- Technical Feasibility & Strategic Project Reports
- Turnkey Manufacturing Facility Solutions
- Regulatory Validation Protocols & Reports
- Controlled Environment Setup & ISO Guidance