Guiding You Through Successful Global Regulatory Audits Navigating regulatory inspections can be one of the most critical and high-stakes phases in pharmaceutical and medical device manufacturing. At Micron, we bring deep expertise in regulatory inspection readiness, support, and response management to help clients meet the expectations of health authorities across the globe.
Our Inspection Experience Covers:
- CDSCO : Central Drugs Standard Control Organization (India). : Support for product registration, plant inspections, and license renewals under Indian regulatory norms.
- USFDA : United States Food & Drug Administration : Guidance on 21 CFR Part 210/211, data integrity expectations, and GMP audit preparedness for sterile and non-sterile product facilities.
- UK-MHRA : UK Medicines & Healthcare Products Regulatory Agency : Assistance in inspections for both pharmaceutical and medical device facilities, covering EU GMP compliance.
- State FDA Inspections (India) : Support for obtaining manufacturing licenses, site audits, and compliance with state-level norms.
- Malta Medicines Authority : Inspection assistance for EU market access and regulatory documentation compliance.
- Agency for Medicinal Products & Medical Devices of the Republic of Slovenia : Support for site readiness, documentation, and regulatory filings for medicinal product exports to the EU.
“Strategic Support Across the Entire Regulatory Lifecycle”
At Micron, we provide specialized regulatory consulting services to help pharmaceutical, biopharmaceutical, veterinary, and medical device companies meet stringent global regulatory requirements. From dossier preparation to post-inspection remediation, our experts offer comprehensive guidance aligned with the latest regulatory frameworks from USFDA, EU, CDSCO, MHRA, WHO, and other international agencies.
Dossier Preparation & Regulatory Submissions
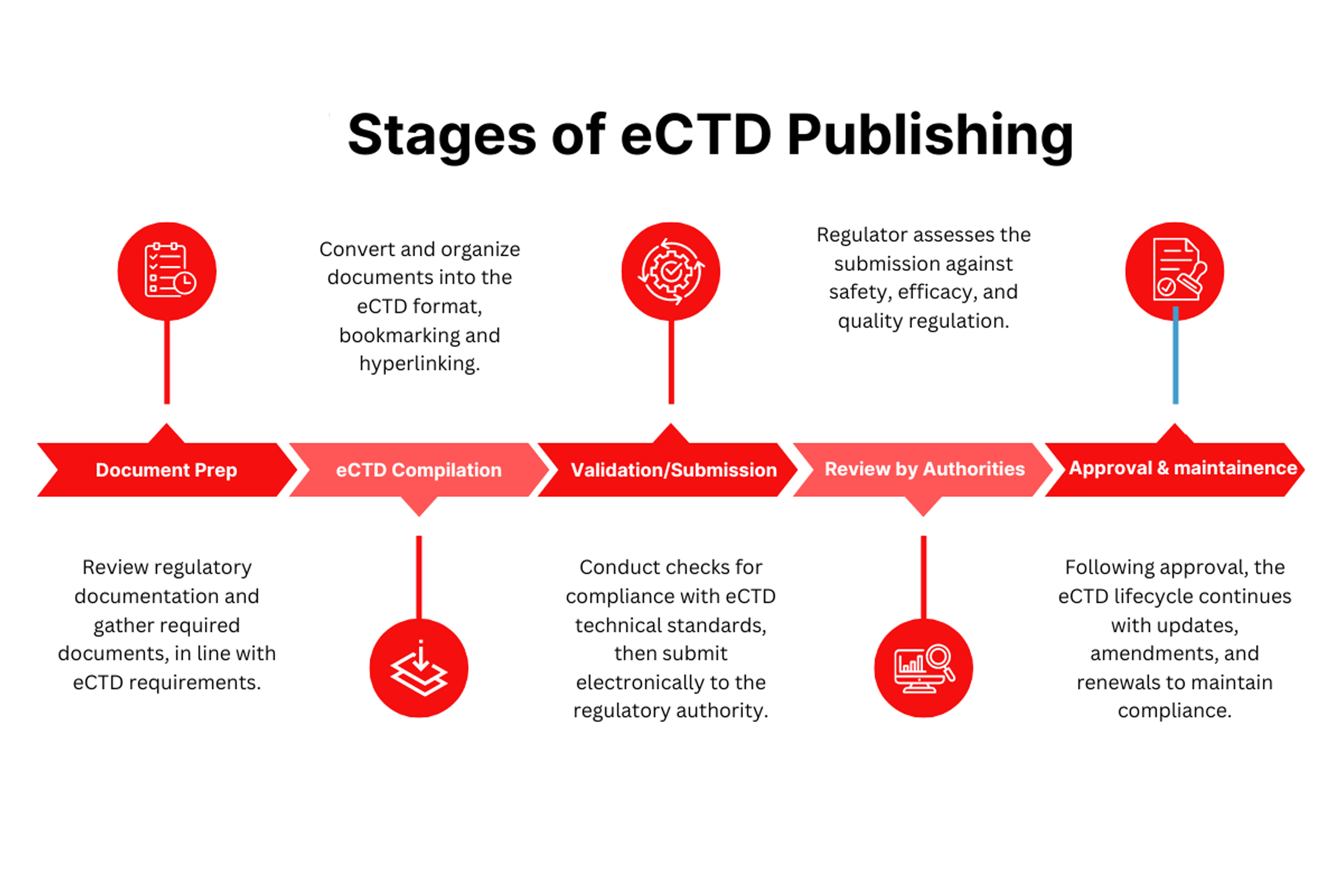
- ANDA (Abbreviated New Drug Application) Submission Support : End-to-end assistance with compiling, reviewing, and submitting ANDA dossiers in eCTD format, including modules 1 to 5.
- DMF (Drug Master File) Compilation & Review : Preparation and review of Type II DMFs, ensuring alignment with ICH and USFDA requirements for APIs, intermediates, and excipients.
- CEP (Certificate of Suitability) Support : Assistance in preparing and submitting applications for EDQM’s CEP certification for APIs entering the European market.
- Product Registration & Licensing Support : Guidance for national and international market authorization applications (MAA), including coordination with CDSCO, WHO PQ, and EU regulatory bodies.
Ensuring Sterility, Safety, and Compliance
At Micron, we specialize in setting up fully compliant, inspection-ready facilities tailored for pharmaceutical, biotechnology, veterinary, and medical device manufacturers. Our expert team works closely with clients to design, implement, and optimize facilities that meet global regulatory Standards.
Quality Control (QC) & Quality Assurance (QA) Department Setup
- Design and implementation of QC/QA departments aligned with GMP and ICH Q10 requirements
- SOP development, equipment selection, and personnel training.
Microbiology Laboratory Setup
- Layout design and setup of clean microbiology labs
- Support in selecting suitable equipment (e.g., laminar airflow, incubators, autoclaves)
- Environmental monitoring design and aseptic area support
- SOPs and validation protocols for microbial testing (MLT, BET, sterility, GPT)
Injectable Facility Setup
- Specialized support for sterile/aseptic injectable facilities (liquids, lyophilized products)
- Layout planning as per ISO 14644 and GMP Annex 1 standards
- Assistance in cleanroom classification (Grade A/B/C/D), HVAC design, and utility mapping
- Guidance on media fill protocols, sterility assurance levels, and process validation
Review of Facility Layout for Finished Dosage Forms
- Evaluation and optimization of facility layouts for OSDs (Oral Solid Dosages), liquids, topicals, and more
- Ensure unidirectional flow of personnel, material, and waste
- Identify and mitigate cross-contamination risks
- Alignment with GMP and cGMP architecture principles
Validation Protocol & Report Review
Preparation and review of IQ, OQ, PQ documents for critical equipment and utilities
- Review of cleaning validation, process validation, and analytical method validation protocols
- Audit-ready documentation aligned with regulatory submission expectations.
Development of QMS & Pharmaceutical Quality System
- Design and implementation of a robust Quality Management System (QMS)